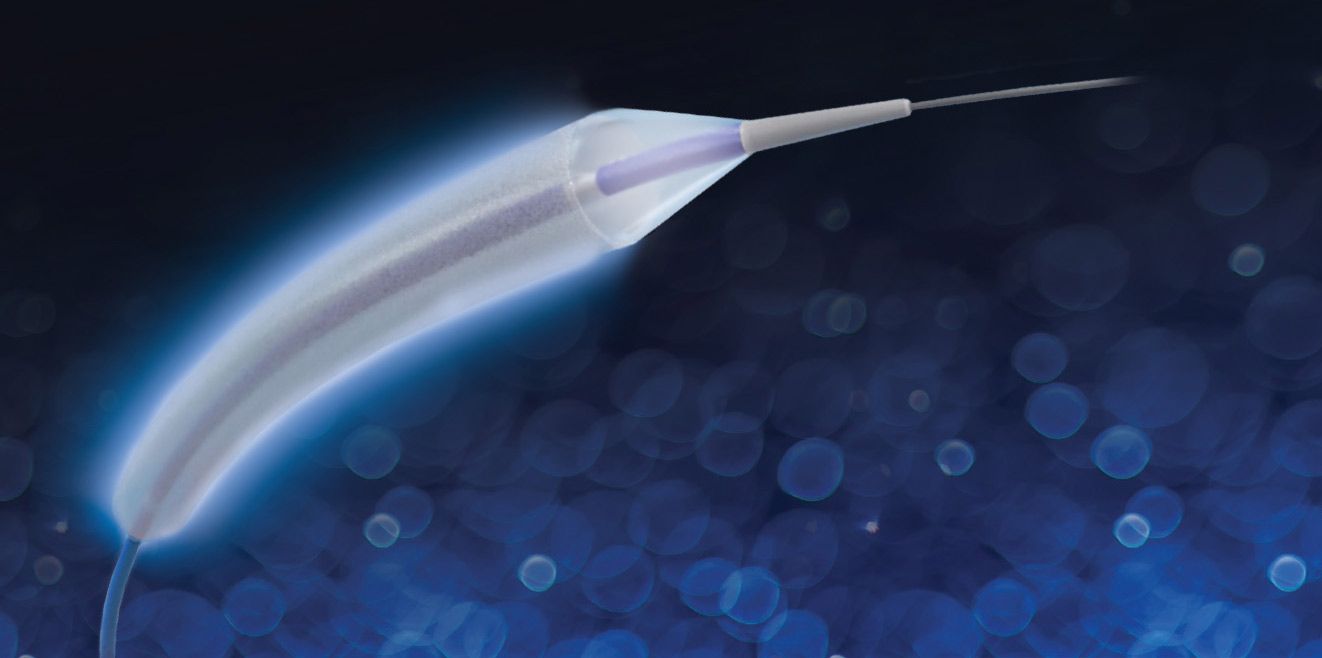
MedAlliance specializes in the development of groundbreaking technology and the commercialization of advanced drug-device combination products for the treatment of coronary and peripheral artery disease. Its flagship drug-eluting balloons (DEB) SELUTION SLR obtained CE Mark approval in Europe and became the first device to be awarded FDA Breakthrough designation for use in Below-the-Knee (BTK) disease.
BTK disease represents the worse part of the spectrum of peripheral artery disease and patients are at an increased risk of limb loss and mortality. One of the cornerstones of BTK treatment is to re-establish blood flow to the foot to promote wound healing. Although percutaneous lower limb angioplasty has become the favoured option of revascularization, its Achilles Heel is vessel recoil and restenosis from neointimal hyperplasia.
SELUTION SLR for treating BTK diseases
MedAlliance announced the completion of patient enrolment in the PRISTINE clinical trial with SELUTION SLR™ 018 DEB for the treatment of patients with BTK. PRISTINE is a Prospective Registry to Investigate the Safety and efficacy of Treatment with SELUTION SLR Sirolimus Drug-Coated Balloon in TASC C and D athero-occlusive Infra-iNguinal disease in patients with chronic limb-threatening ischemia from SingaporE.
The objective of this trial is to evaluate the safety and efficacy of the SELUTION SLR DEB in the treatment of infra-inguinal occlusive lesions (TASC C and D) in patients with chronic limb-threatening ischemia in 75 patients over 12 months at Singapore General Hospital.
PRISTINE is a follow-up registry to the PRESTIGE Trial. The 12-month data from PRESTIGE was presented at LINC 2021 in January, showing sustained benefits up to one year. 18-month data is to be presented at VIVA in October this year, where it is anticipated that these benefits will be further sustained. A similar outcome benefit is expected from PRISTINE in a larger real-world population.
Certified in Europe
In February 2020 MedAlliance received CE Mark approval for SELUTION SLR in the treatment of PAD and in May 2020 received CE Mark approval for treatment in Coronary Artery Disease (CAD). MedAlliance has been awarded FDA Breakthrough designation for the SELUTION SLR for use in BTK. The company – with offices in Germany, Singapore, UK and the USA – expects to begin the IDE study later this year. The company secured CHF 50 million early this year to fund and commercialise SELUTION SLR and support global clinical programs.
(Press release/RAN)























































Please login or sign up to comment.
Commenting guidelines