Medtech company MedAlliance has signed an exit agreement with Cordis, a worldwide leader in interventional cardiovascular and endovascular technologies present in 70 countries. The acquisition includes an initial investment of $35M and an upfront payment of $200M upon closing in 2023. The total transaction may result in up to $1.135 billion. Cordis will immediately begin co-promotion of MedAlliance’s SELUTION SLR drug-eluting balloon in markets where it is commercially available.
Cordis, a global developer and manufacturer of interventional cardiovascular technologies, has entered an agreement to acquire MedAlliance, to strengthen its position in Drug-Eluting Balloon Technology, In addition to the investment and upfront payment, MedAlliance will receive regulatory achievement milestones of up to $125M and commercial milestones of up to $775M through 2029, bringing the total transaction to 1.135 Billion. Cordis will immediately begin co-promotion of MedAlliance’s SELUTION SLR drug-eluting balloon in markets where it is commercially available.
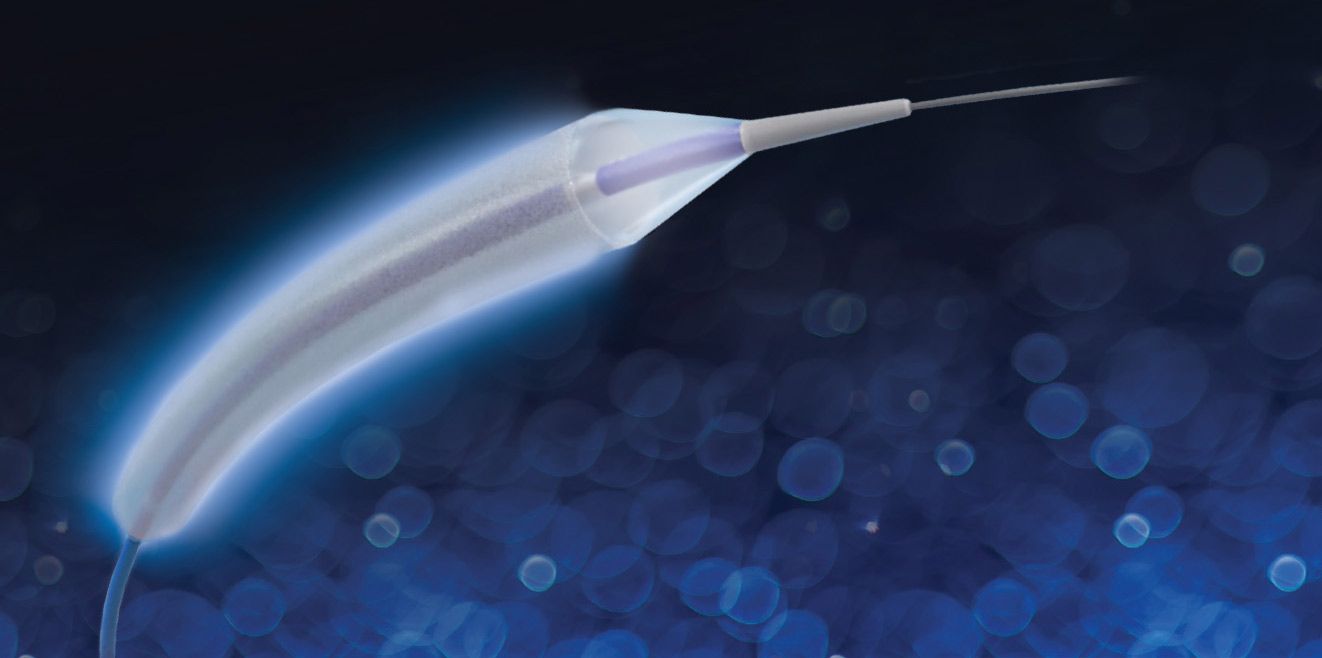
Founded in 2008 and based in Nyon, MedAlliance focusses on drug-device combination products, initially for treating coronary and peripheral artery disease. Its flagship product SELUTION SLR (Sustained Limus Release), is a novel drug-eluting balloon platform technology that leverages spherical MicroReservoirs made from biodegradable polymer intermixed with the proven anti-restenotic drug sirolimus. These MicroReservoirs provide controlled and sustained drug release for up to 90 days, making SELUTION the only drug-eluting balloon using MicroReservoirs and providing the most effective pharmacokinetics release profile of any device on the market.
Approximately 10,000 patients have benefitted from SELUTION, including 9,200 commercial units and over 2,000 patients treated in clinical trials. The company has also enrolled more than 500 patients of the 3,326 planned in the coronary randomised controlled study comparing SELUTION SLR with any limus drug-eluting stent (DES), powered to demonstrate the superiority of SELUTION SLR DEB over DES. SELUTION DeNovo is the largest DEB study ever initiated and has the potential to change medical practice. Cordis customers will benefit from the extensive clinical study program and publication plan that MedAlliance is executing.
SELUTION SLR was awarded CE Mark Approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020. The US FDA has also awarded SELUTION SLR with four breakthrough designations. Coronary, BTK and SFA indications have received FDA IDE approval and IDE clinical studies are currently enrolling.
(Press release/RAN)























































Please login or sign up to comment.
Commenting guidelines