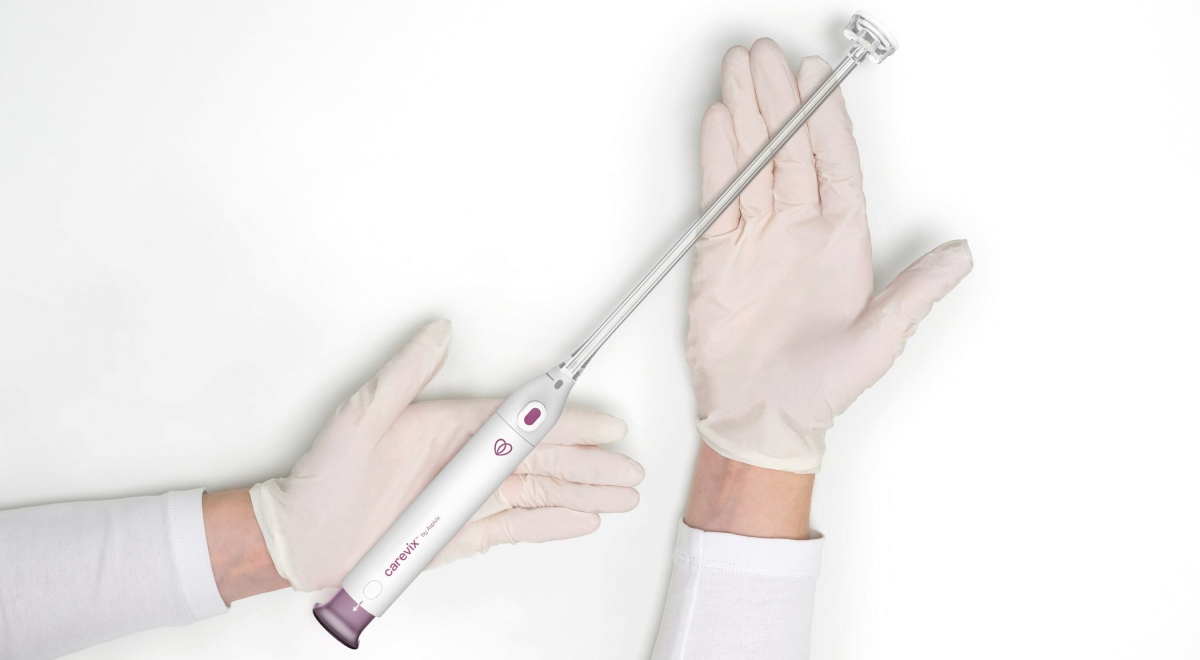
With the CE Approval of Carevix by Aspivix, more women now have access to less painful procedures during Intrauterine Device placement and other gynaecological procedures in the US and Europe. Ambassadors across 12 institutions in Europe and the US are already using the product.
Aspivix is a pioneering leader in women’s health, dedicated to modernising healthcare solutions for women around the world. Its flagship product Carevix is a cervical stabiliser that uses a gentle approach to minimise pain and bleeding during various transcervical procedures, including Intrauterine Device (IUD) placement. Carevix is the result of the constant commitment atASPIVIX to make gynecology, now modern.
Carevix™ has been clinically proven to be atraumatic during transcervical procedures. ADVANCE Women, a single-blinded, randomised, multicentric, comparative study was conducted on 100 women who underwent an IUD insertion with either the Carevix device or a traditional cervical tenaculum. Results showed that women who underwent IUD placement with carevix reported statistically and clinically significant results with up to 73% reduction of pain scores and 78% reduction in bleeding occurrence rates. The results have been published in “Contraception”, the International Reproductive Health Journal in March 2023.
In addition to the 510 (k) FDA clearance, Carevix has now obtained the CE-Mark approval, making it available for commercialisation in Europe and the US. This CE mark approval allows ASPIVIX to expand worldwide and collaborate with ambassadors (OBGYNs, midwives, nurses) across 12 centers of excellence in the US, France, Sweden, Switzerland, Germany, and Austria. The ambassadors are currently using Carevis in their routine, gaining invaluable insights and providing women with a better experience in gynaecology.
(Press release)























































Please login or sign up to comment.
Commenting guidelines