The CAREVIX cervical device by ASPIVIX will ensure a gentler gynaecological procedure for women considering an intrauterine device as a contraception option. Indeed, interim results from the first-in-women-trials for the device confirmed its safety, efficacy and usability.
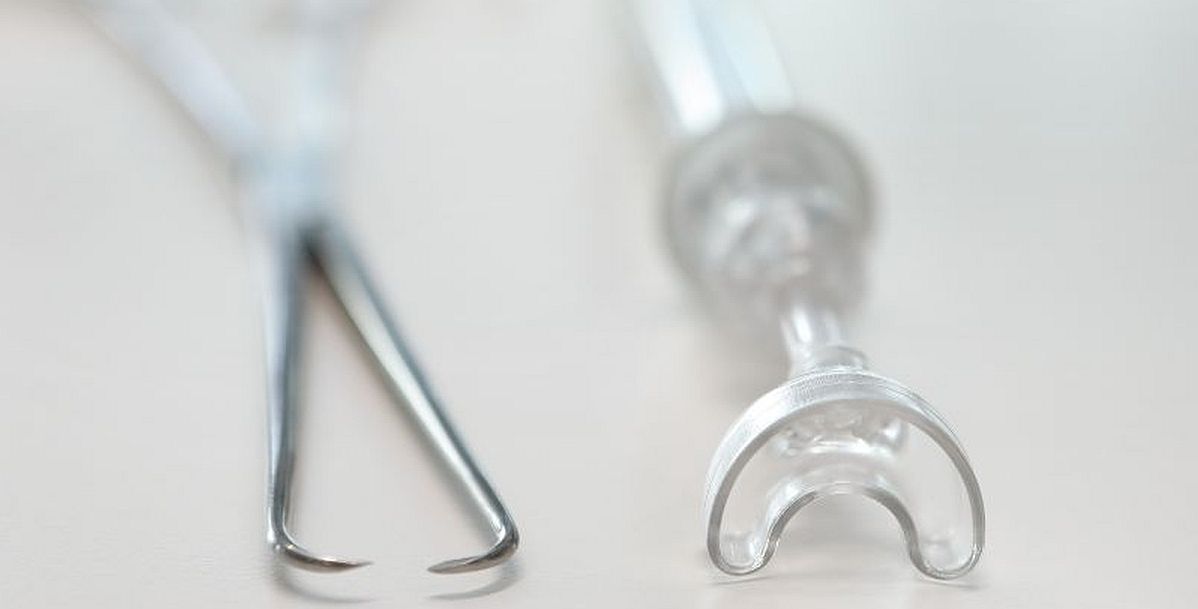
The intrauterine device (UID) is the most cost-effective and reversible contraceptive solution. However, more than 80 per cent of the women conspiring this alternative experience pain, bleeding and tissue tear during IUD placement due to the tenaculum, a forceps-like surgical instrument used in the procedure. ASPIVIX promises to advance gynaecological care and women’s healthcare with its CAREVIX. The vacuum-based cervical device is an innovative, soft-suction cervical device is designed as a modern and gentler alternative to a tenaculum.
The medtech startup has now completed its first-in-women trial; the ADVANCE Women (Atraumatic Device using VAcuum Technology for CErvical Procedures in WOMEN) Phase I trial, conducted at Lausanne University Hospital (CHUV) by Patrice Mathevet, an expert in innovative gynaecological surgical approaches, and his team.
The two-phase trial assessed the usability, efficacy and safety of the investigational CAREVIX. Interim results suggest that women experienced lower procedural pain than published literature from IUD procedures using a tenaculum. Moreover, there was no procedural bleeding or tissue tear related to the CAREVIX device.
“We are pleased to gather positive initial physician and patient experience using the CAREVIX device. We believe it has the potential to transform how IUD procedures are performed and to motivate more women to consider it”, said Mathieu Horras, CEO of ASPIVIX. “We look forward to conducting the ADVANCE Women Phase II trial, a randomized, multi-centre study comparing pain scores and other complication rates among women receiving an IUD with CAREVIX versus procedures using a tenaculum.”
Based in Renens, ASPIVIX’s innovative technology, development and clinical program are supported by investors 4FO Venture Partners and Zürcher Kantonalbank (ZKB), European Union research and innovation program Horizon 2020, and visionary angel investors.
(Press release/RAN)
Photo: Traditional cervical tenaculum (left) and CAREVIX (right) - Zuzanna Adamczewka-Bolle























































Please login or sign up to comment.
Commenting guidelines