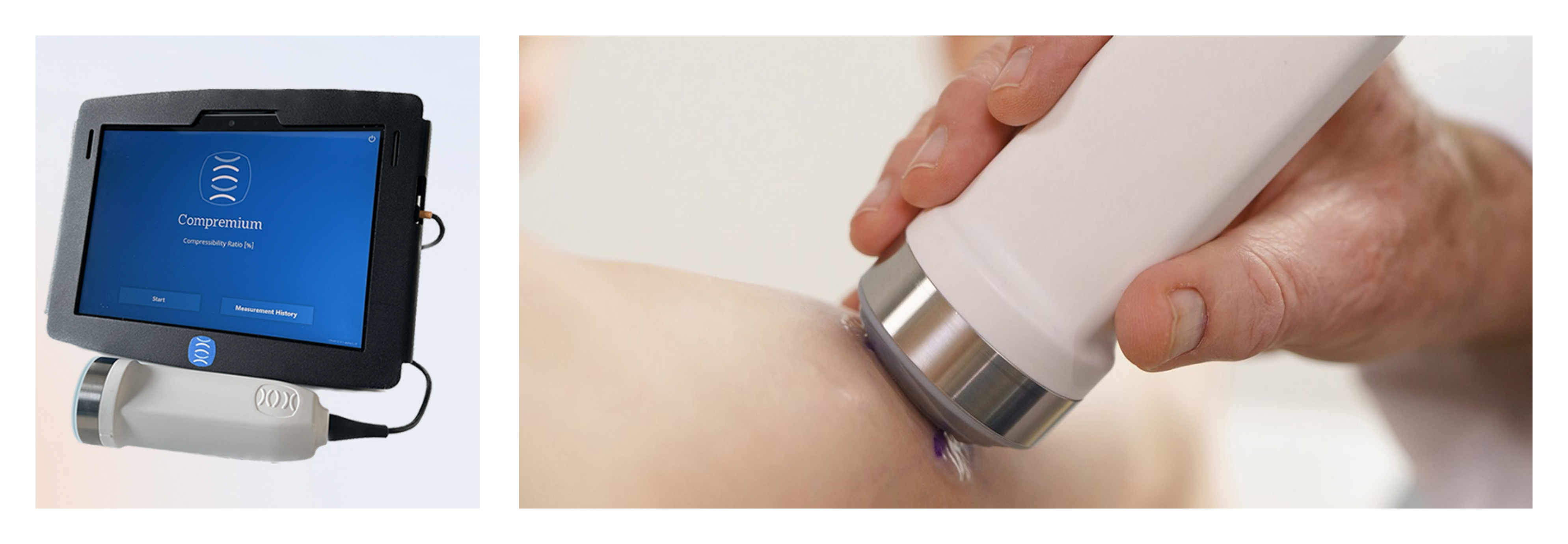
Early this year, Compremium received FDA Clearance for its Compartmental Compressibility Monitoring System (CPMX1), the first medical device capable of measuring the compressibility of muscles and tissue in the human body. The startup has now delivered the first series to NASA.
Compremium’s Compartmental Compressibility Monitoring System (CPMX1) enables healthcare professionals to measure and monitor pressure-related health conditions non-invasively and reliably for the first time. The device provides objective muscle or tissue compressibility values related to trauma, emergencies, or chronic conditions. Data obtained with the help of the CPMX1 is of high importance and can aid in reducing the number of missed and delayed diagnoses and eliminate overtreatment due to uncertainties in the clinical evaluation. The CPMX1 System has proven to be a precise, reliable diagnostic aid in multiple clinical studies.
Following two years of development and validation, the Bern-based medtech company received approval from the US Food and Drug Administration (FDA) for real-time and intermittent monitoring of relative compartment compressibility in April, paving the way for applying it in clinical practice.
Compremium will deliver the first product series to NASA’s Johnson Space Center in Houston (Texas), which aims to apply the CPMX1 in deep space missions to the Moon and Mars to understand the shifting of fluid in the human body under weightlessness. The device can detect changes in the body resulting from prolonged exposure to zero-G and will be used to ensure astronaut health during long flights in zero gravity. This mission will start in 2024.
“Today’s shipment marks a significant milestone in our journey to support NASA in their mission. Not only will this medical device enhance astronaut safety, but the knowledge gained from this project will also have far-reaching benefits for healthcare on Earth.,” states the startup in its LinkedIn post.
Long-term relationship
The partnership between NASA and Compremium dates back over 20 years when Ulrich A. Baumann (Chief Medical Officer at Compremium) set out to develop a revolutionary device, the Veinpress device, to measure venous pressure in the human body non-invasively. He built the prototype, followed by initial studies in 2013 at the NASA laboratory in Houston and intensive tests in parabolic (zero gravity) flights with NASA crew members out of Bordeaux in France. The Veinpress device has been used on the International Space Station (ISS) since 2018, and various studies have been published since then. The Veinpress is the precursor of the CPMX1, the latest device from Compremium.
Compremium continues to advance its platform technology through collaborations with scientific partners, institutions, and reputed hospitals worldwide.
(Press release/RAN)























































Please login or sign up to comment.
Commenting guidelines