Compremium, CoreMedic, Cutiss, Comphya and Novostia are making strides in the field of healthcare. Their solutions, addressing critical medical challenges from heart valve diseases to grave skin defects and erectile dysfunction, are currently being tested in humans.
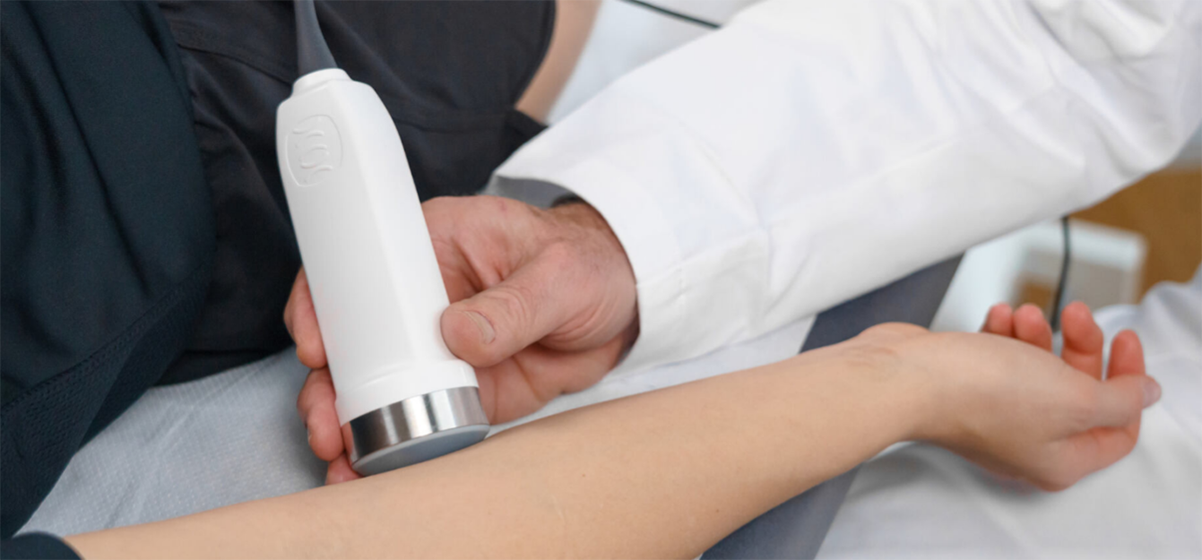
Based in Canton Berne, Compremium is developing diagnostic solutions for pressure-related conditions in the human body. Its platform technology is a non-invasive, portable and ultrasound-based solution, potentially making traditional invasive measurements of suspected ACS obsolete. In a recent study with 25 pediatric patients, the company’s pressure sensor, following the pressure-related ultrasound – PrUS – approach, was tested and showed promising results in measuring and managing soft tissue swelling after limb fractures. Swellings in a body’s anatomical compartment can lead to insufficient blood supply to tissue within that space, which poses a risk for a patient to develop acute compartment syndrome (ACS). The pressure sensor will have to be tested in larger cohorts to validate its practicality and performance in daily clinical settings.
Cardiology company CoreMedic, based in Switzerland and Germany, announced the first successful transcatheter application of their ChordArt technology (ChordArt TMVr-System) in a First-in-Human trial for a patient suffering from severe degenerative mitral valve regurgitation (MR). The mitral valve leaflets do not close properly in patients with MR, causing blood to leak backwards across the valve, eventually leading to a shortage of blood moving to the rest of the body. MR is the most common type of heart valve disorders, affecting more than 25 million people worldwide. The ChordArt device is designed to enable safe and effective repair of the mitral valve by replacing damaged mitral valve chords with a conservatory physiological approach. CoreMedic is planning the next trial with more patients suffering from MR in a few months.
Schlieren-based startup Cutiss, which focuses on skin regenerative medicine and tissue engineering, just closed the recruitment process for their denovoSkin phase II clinical trial in reconstructive surgery for children and adults. Conducted in Swiss, Italian and Dutch hospitals with 20 patients (one year and older), the study will test the safety and efficacy of a personalized, bio-engineered skin substitute – denovoSkin. The skin product is bio-egineered in large quantities originating from a post stamp sized piece of healthy skin. With a follow-up period of 3 years for patients, the first results could already be available within the next 12 months. In addition to this reconstructive surgical clinical trial, denovoSkin in adult and adolescent burn patients is currently transitioning towards phase III clinical trials in Switzerland and the EU, with Orphan Drug Designation for the treatment of burns from Swissmedic, European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA).
EPFL spin-off Comphya specializes in the treatment of erectile dysfunction in patients non-responsive to oral therapy. The startup developed and patented CaverSTIM, a neuro-stimulator which, when implanted in the pelvic cavity, will selectively activate the nerve to trigger penile erection. The device will provide self-controlled stimulation (via a wireless remote control) to the cavernous nerve to restore natural erectile function. The stimulator can be remotely operated by external controllers. In October, the startup has announced the first implantation of its CaverSTIM in spinal cord injury individuals. Following this initial success, additional surgeries are ongoing in collaboration with FMABC (Faculdade de Medicina do ABC) at two study centers, the Hospital Mario Covas and the Hospital do Servidor Publico de São Paulo in Brazil.
Novostia is developing an artificial heart valve, TRIFLO, which is a unique valve made of high-performance biocompatible material that allows optimal blood flow, prevents the formation of clots and has great durability, while closing smoothly and silently. The Lausanne-based startup recently received the green light from the Lithuanian State Health Care Accreditation Agency to launch the First-in-Human trial to implant its aortic heart valve prosthesis, TRIFLO. This trial, scheduled to start in December 2023, will take place at Vilnius University Hospital Santaros Klinikos. Since its initiation in 2017, the TRIFLO project has been backed by positive and high-performance data in silico, in vitro and in vivo. The successful TRIFLO valve project is supported and co-financed by the EU through the EIC Accelerator program, by the Swiss Confederation through Innosuisse, and by the Canton of Vaud through the SPEI and the Biopôle.
(Press release/ SR)






















































Please login or sign up to comment.
Commenting guidelines