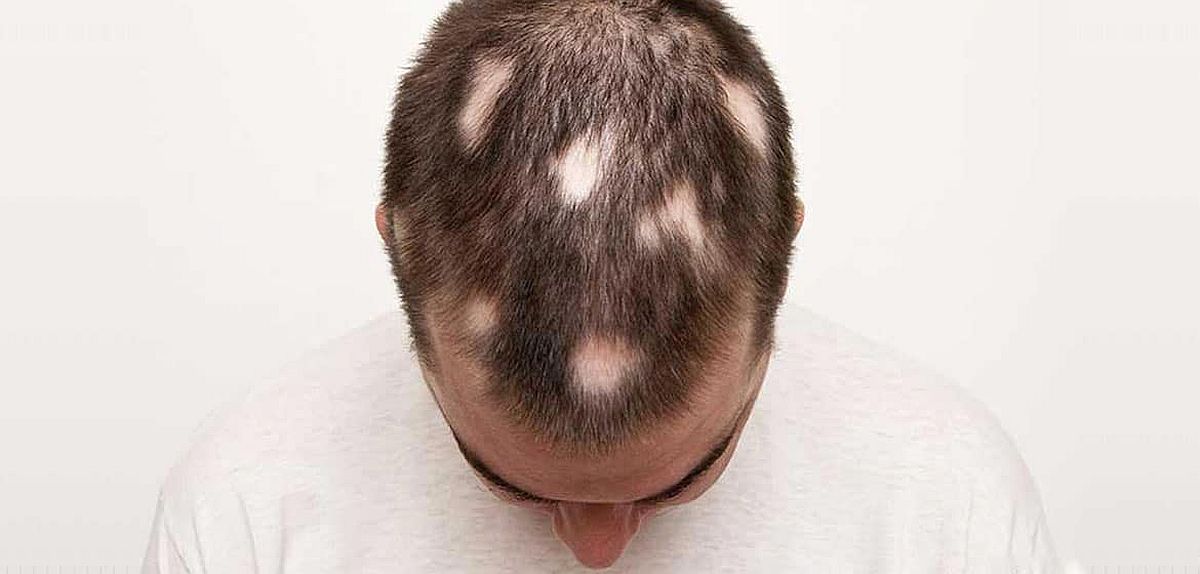
There is currently no approved medication for moderate and severe Alopecia areata, an autoimmune condition causing disfiguring scalp hair loss, in children and adolescents. A drug candidate by Lausanne-based startup Legacy Healthcare that addresses this unmet medical need has shown positive results in a Phase 2/3 study (RAAINBOW).
Legacy Healthcare is a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates with unparallel safety to address the needs of patients with autoimmune and inflammatory diseases such as Pediatric Alopecia Areata, Chemotherapy Induced Alopecia and Cancer Related Fatigue. Its most advanced candidate LH-8 (Coacillium) is the first and only drug candidate for alopecia areata in children and adolescents with both moderate and severe forms of the disease.
Alopecia areata (AA) is a psychologically devastating autoimmune disorder that affects between 2.11% and 2.47% of the population1, including 4.2 million children worldwide, among which 200’000 patients are in Europe and 125’000 in the US. AA is characterized by the loss of patches of hair, all scalp hair or all body hair. With one oral drug recently approved for severe AA in adults, and more in progress, interest and understanding of the disease and its impact have increased. A major key learning is the need for treatments that can be used early enough in the course of the disease, while it is still Moderate, to prevent, delay, or reverse the progression to a severe stage, as well as treatments that are safer and better tolerated, and treatments which discontinuation does not result in rapid disease relapse.
Legacy Healthcare has successfully completed its RAAINBOW phase 2/3 randomized placebo-controlled trial evaluating Coacillium 22.25% cutaneous solution in children and adolescents with moderate and severe alopecia areata. Results showed that patients presenting moderate AA improved under Coacillium treatment, while the control group of patients receiving a placebo worsened to a more severe stage of the disease.
To evaluate potential disease relapse, patients were followed for a period of 6 months after treatment discontinuation. Almost all patients whose alopecia areata improved during the 6-months treatment with Coacillium kept improving after treatment discontinuation, suggestive of a direct long-term action of Coacillium on the physiopathological process causing alopecia areata.
No serious adverse events (AEs) were reported. One adverse event was considered as definitely related to Coacillium (acute scalp and face eczema), and three probably or possibly related. All AEs were cutaneous, mild or moderate, and transient. The excellent safety of the drug observed in the trial was consistent with observations from previous studies. Coacillium is also being evaluated in persistent chemotherapy induced alopecia.
With these new data, we observe that not only Coacillium treatment works in most patients who use it, but its benefit is maintained after treatment discontinuation - at least during the period of observation of 6 months. This would represent a significant change in treatment paradigm, for patients, physicians and stakeholders" said Saad Harti, Chief Executive Officer of Legacy Healthcare.























































Please login or sign up to comment.
Commenting guidelines