Following the dosing of the first patient in its phase II study of lead candidate, ESO-101, for treating upper gastrointestinal tract diseases, EsoCap secured fresh capital to scale activities. The funds stem from existing and new investors to support the startup’s activities.
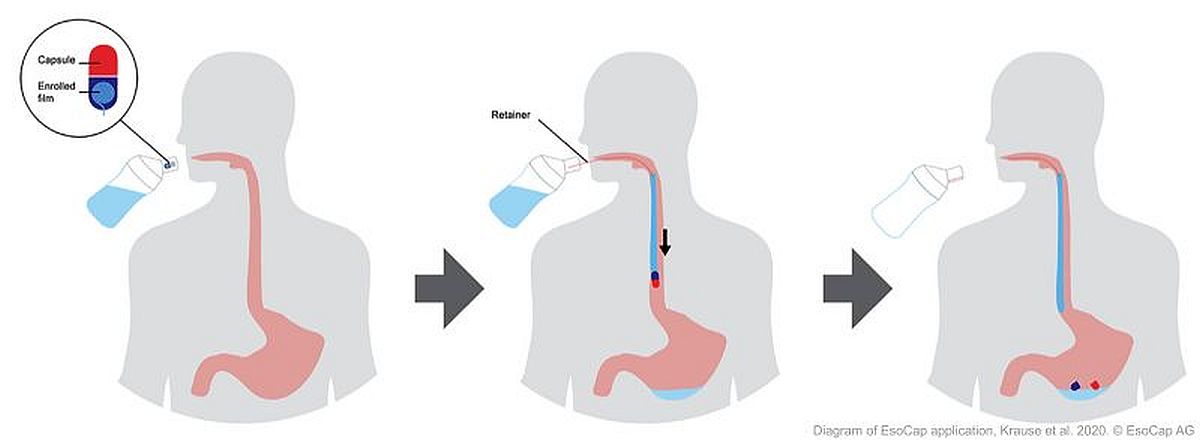
Based in Basel, EsoCap developed a proprietary and unique targeted application platform to increase mucosal contact time and esophageal drug deposition. The esophagus is a muscular tube that connects your mouth and your stomach. The EsoCap system is a unique drug delivery system for the upper astrointestinal tract, consisting of a capsule containing a thin mucoadhesive film loaded with an active pharmaceutical ingredient (API).
The first product candidate, ESO-101, is loaded with mometasone furoate, an anti-inflammatory corticosteroid and well-known active substance approved as a topical treatment for asthma and skin conditions. It is thus well suited as a locoregional treatment for Eosinophilic esophagitis (EoE). Upon drinking the capsule from a specially designed drinking cup, the film unrolls and sticks to the patient’s inner lining of the esophagus - the esophageal mucosa -, where it dissolves slowly, while releasing mometasone furoate. With this solution, EsoCap became the first company to enable local therapy in the esophagus. ESO-101 has received Orphan Drug Designation from the FDA in the treatment of EoE.
In a previous trial, EsoCap’s was shown to increase mucosal contact time dramatically and was well accepted. The ongoing Phase II trial is a randomized, placebo-controlled, double-blind trial that will evaluate the efficacy, tolerability and safety of ESO-101 in 42 adult patients with active EoE in four European countries. Patients will be randomized 2:1 and will be treated for 28 days.
The startup has recently completed a private financing round, raising funds from existing and new investors. The new funding was facilitated by EsoCap benefitting from a unique programme implemented by the Canton of Basel-Stadt, guaranteeing subordinated loans to support technology startups during the Covid-19 pandemic. The proceeds will be used to for the development and industrial scale-up of activities for EsoCap’s targeted application technology, enabling effective local treatment of esophageal diseases for the first time.
“We are delighted to have secured the financial resources to pursue our rapid development. We plan to use the proceeds to progress the ACESO phase II clinical study in eosinophilic esophagitis (EoE), to pursue the feasibility of its technology platform for other esophageal diseases with a high level of unmet medical need, including Barrett’s Disease and reflux disease, and to ramp up industrial scale-up.”, said Isabelle Racamier, EsoCap AG CEO.
About EoE
Approximately half a million patients worldwide (5 in 10,000) suffer from Eosinophilic esophagitis (EoE), a rare, chronic, local immune-mediated esophageal disease characterized clinically by symptoms related to oesophagal dysfunction, and histologically by eosinophil-predominant inflammation. The symptoms of EoE include swallowing disorders, food impaction, vomiting, and heartburn. EoE is the leading cause of dysphagia and food impaction in children and young adults.
Existing treatments include extremely strict diets, off-label treatment with steroids, or off-label proton pump inhibitors, or an orodispersible budesonide tablet available only in limited territories. These options remain suboptimal for the vast majority of affected patients.
(Press release / SK)























































Please login or sign up to comment.
Commenting guidelines