Augurix in cooperation with GaDia and their partner in Asia, is launching the first rapid serological screening test for COVID-19 on the Swiss market. This innovative technology opens up the prospect of a large-scale evaluation of the population.
The rapid test has been co-developed by Augurix and GaDia on the basis of SARS-Cov 1 and on the experience gained during the validation of previous rapid tests such as the detection of infectious and nosocomial diseases or celiac disease, and has obtained the CE mark. Augurix, will initially distribute the test kit to doctors' surgeries, private and public hospital laboratories, as well as to pharmacy staff and all the country's healthcare personnel.
In a second phase, depending on the authorizations granted by the regulatory authorities, an extension of the availability of this rapid test may be offered to pharmacy customers and the general population, as well as for export.
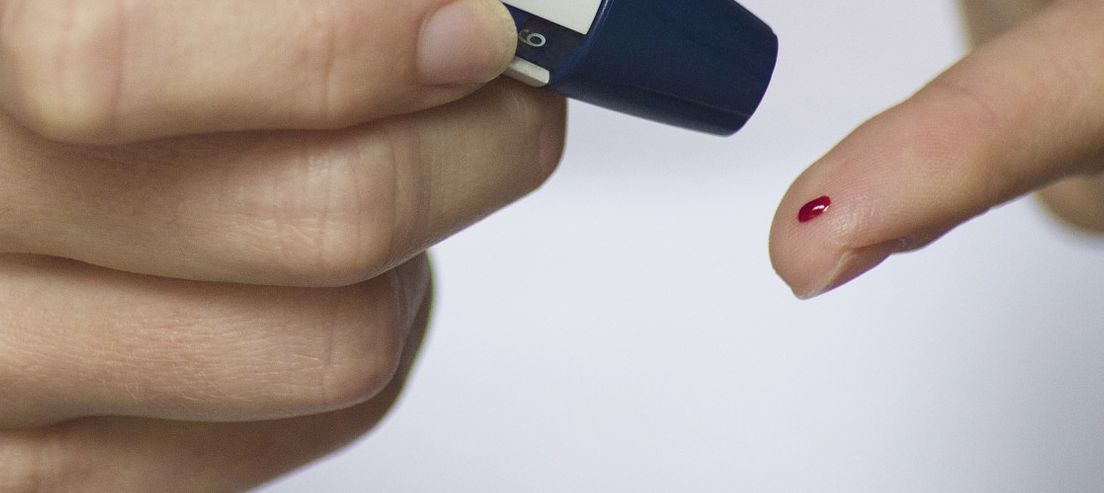
Results after 10 minutes
A single drop of blood from the fingertip deposited in a device the size of a pregnancy test indicates after 10 minutes the presence of specific IgM and/or IgG antibodies that develop in a CoV2-SARS infection (COVID-19). A positive result given by this rapid test indicates that the patient has been infected with the coronavirus and has developed antibodies specific to the SARS-CoV-2 virus causing COVID-19 infection, whether or not the patient has shown symptoms of the disease.
Enabling mass testing
This innovative technology opens up the prospect of a large-scale evaluation of the population's immunity to the SARS-CoV-2 virus, at a modest cost compared to conventional serological analyses and requiring a minimum of staff resources for its implementation.
The Microbiology Laboratory of the CHUV (University Hospital Lausanne), will participate in the quality control and is currently evaluating this test for its internal needs. According to studies of more than 1,000 cases to date, the Augurix COVID-19 test has a sensitivity of 98.5% for a specificity of 96.0%.
(Press release / SK)























































Please login or sign up to comment.
Commenting guidelines