Novostia has just announced the closing of its Series A financing round with 2.5 million euros that will enable the launch of First-in-Human (FIH) trial. A Series B round will be opened this summer, after re-evaluation of the company to complete the Pilot Study and launch in its wake the Pivotal Study.
Novostia SA, a clinical stage company developing a breakthrough artificial heart valve, has just raised an additional EUR 2.5 million. The total funds raised since its foundation in April 2017 in Neuchâtel, amount to just over 15 million and include public subsidies from the European Union and the Canton of Vaud. Novostia is based at Biopôle close to Lausanne and led by Alain Barbat.
The Series A round which has now been closed enables the launch of First-in-Human (FIH) trials. A Series B will be opened this summer, after re-evaluation of the company. This Series B financing round will be used to complete the Pilot Study and launch in its wake the Pivotal Study which will enroll around 60 patients for the former and 500 for the latter. These studies will enable Novostia to obtain the necessary approvals (CE mark and Pre-Market Approval) to enter the market.
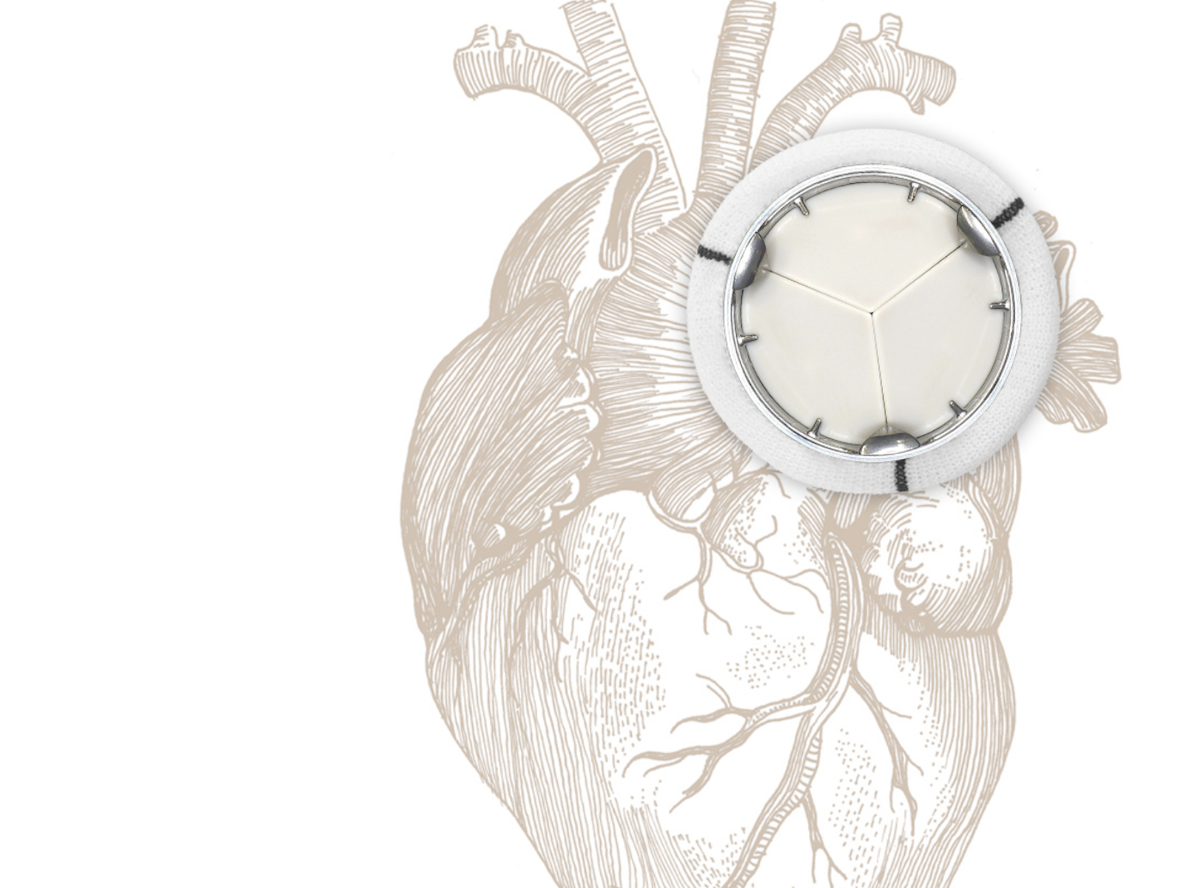
Heart valve diseases affect more than 100 million people worldwide. Every year hundreds of thousands of patients undergo a heart valve replacement. Available technologies entail serious constraints: lifelong anticoagulant medication or further replacements due to limited valve durability.
Thanks to a unique patented design and the use of a high-performance biocompatible polymer, the company led by Alain Barbat eliminates all these constraints. Experimental, numerical and animal testing demonstrates that the valve physiologically operates like a native human heart valve: it does not produce high velocity backflow jets, does not elicit an haemostatic response and thus is expected to function without anticoagulation therapy. Unlike tissue valves, which have limited durability, especially with young patients, the valve is structurally designed to last a lifetime for patients of any age, thereby avoiding the risk of a re-operation.
(Press release / ES)























































Please login or sign up to comment.
Commenting guidelines