Medyria has developed a catheter positioning system with the potential to significantly reduce the use of x-ray and contrast dye. The first clinical trial has started.
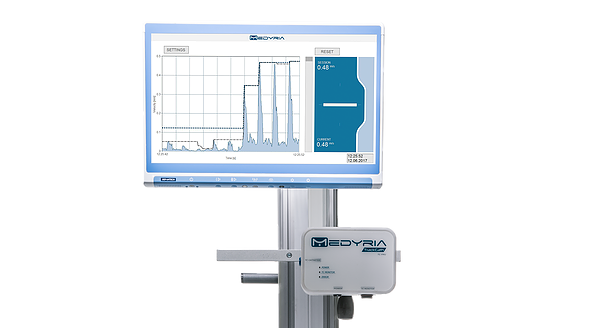
The TrackCath Catheter Positioning System of Medyria consists of a catheter, flow measurement unit and a monitor. A patented sensing technology measures the blood’s velocity and identifies for the accurate positioning and cannulation in the aorta for significantly reducing the use of X-ray and contrast dye during the Endovascular Aneurysm Repair (EVAR) procedures.
Reducing the amount of contrast dye injected in chronic kidney disease (CKD) patients is aligned with cardiology and radiology society guidelines that urge physicians to use dye sparing approaches in patients at risk of Contrast-induced acute kidney injury (CI-AKI). Prevention of CI-AKI may lead to shorter hospital stays, improved patient outcomes, and may ultimately save patients’ lives.
The first in man clinical trial has started. The Germany based 32 patients multi center clinical trial held in UKD, Dusseldorf (Principal Investigator and study coordinator , Prof, Hubert Schelzig), UniKlinik Aachen (Dr. J. Kalder), UnliKlinik Leipzig (Dr. A. Schmidt) and UKE Hamburg (Prof. T.Koelbel), has enrolled and treated 27 EVAR patients so far.
"I believe that this product can reduce the use of contrast dye and reduce the radiation to patient and to the surgeons. The trackCath system appears to be of great help for the cannulation of visceral arteries in complex cases. I am looking forward to see the outcomes of the multi-center first in man ACCESS study." said Dr. Andrej Schmidt, principal investigator from Leipzig.
(Press release - SK)























































Please login or sign up to comment.
Commenting guidelines