One year following the receipt of its first FDA clearance for its miniature hip navigation technology, NaviSwiss has obtained a green light to market its Naviplan, an advanced planning solution for hip replacement surgery, in the U.S market.
As the technology leader in miniaturized surgical navigation solutions, Brugg-based medtech startup Naviswiss supports orthopaedic surgeons in accurately positioning hip replacement implants. Besides yielding immediate results, simplifying workflows, and improving quality the solutions help to minimize the time and cost of surgical procedures.
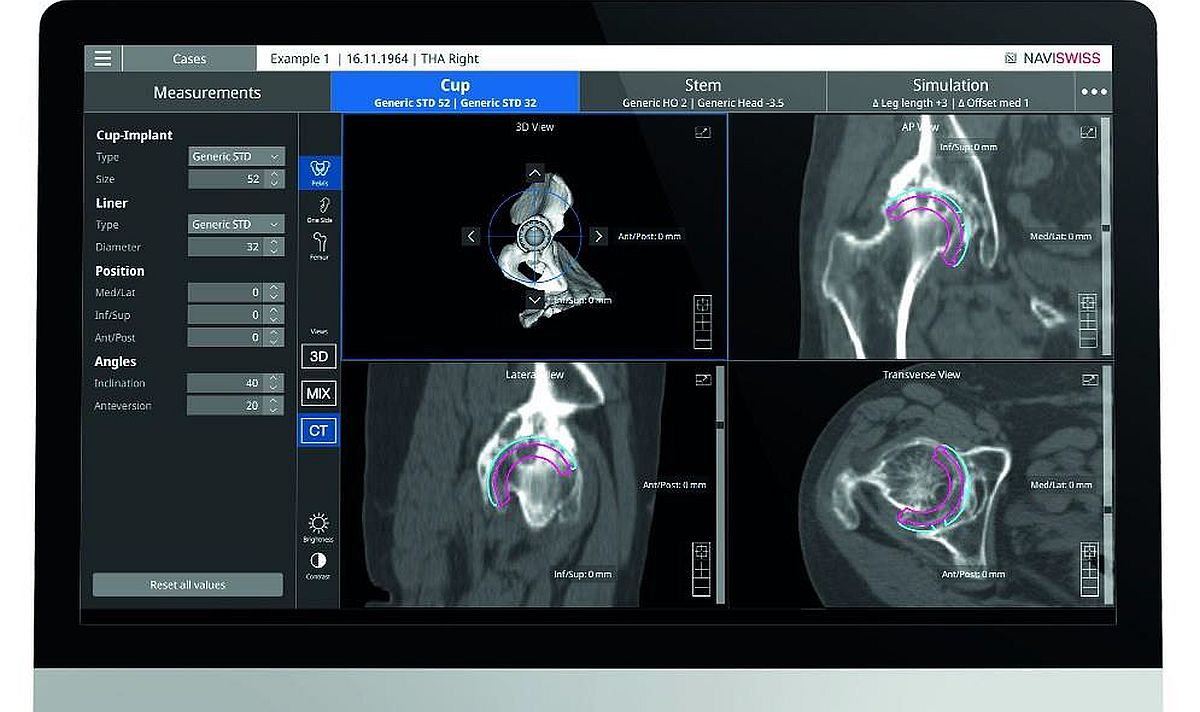
Its product Naviplan is a digital pre-operative planning solution that enables orthopaedic surgeons to perform navigated CT-based total hip replacement surgery. It assists the surgeon in the optimal positioning of the joint implants, automatic 3D segmentation and advanced image processing. It has the potential to improve accuracy and predictability and provide seamless documentation of the outcome.
The new FDA clearance for Naviplan marks an important step for the company to bring the solution into the market. Naviswiss is releasing the CT-based Navigation and Naviplan to orthopaedic care centers in the United States over the course of the fourth quarter of 2021.
"Naviplan and CT-based Navigation are an important addition to the Naviswiss portfolio and complete our offering for navigated hip replacement”, said Jan Stifter, Naviswiss CEO. “We now have two patient-specific options where the surgeon determines the best application for the procedure. CT-based Navigation may be needed in difficult deformity cases while kinematic registration may be preferred in more traditional surgeries. The surgeon can rely on highly accurate guidance in placing the acetabular components.”
Hip Navigation system in use in the U.S
Its first product the miniature hip navigation system uses a proprietary tracking technology with 95% smaller trackers to provide real-time measurements down to the degree for anteversion, inclination, leg length, and offset. It is an open platform that works with all major hip implants and approaches.
The Swiss-made Naviswiss system reduces exposure to radiation with imageless technology, and it can be easily transported between operating rooms, supporting multiple procedures. In addition to providing intra-operative measurements down to the degree, the system also documents the final implantation parameters that allow surgeons and patients to review the final surgical results.
Last year in June, NaviSwiss received the first FDA clearance for the hip navigation technology and a few months after the clearance, it had the first successful U.S. surgery conducted with its hand-held hip navigation system in New York City, NY. This marked the launch in the US Market.
(Press release/RAN)























































Please login or sign up to comment.
Commenting guidelines