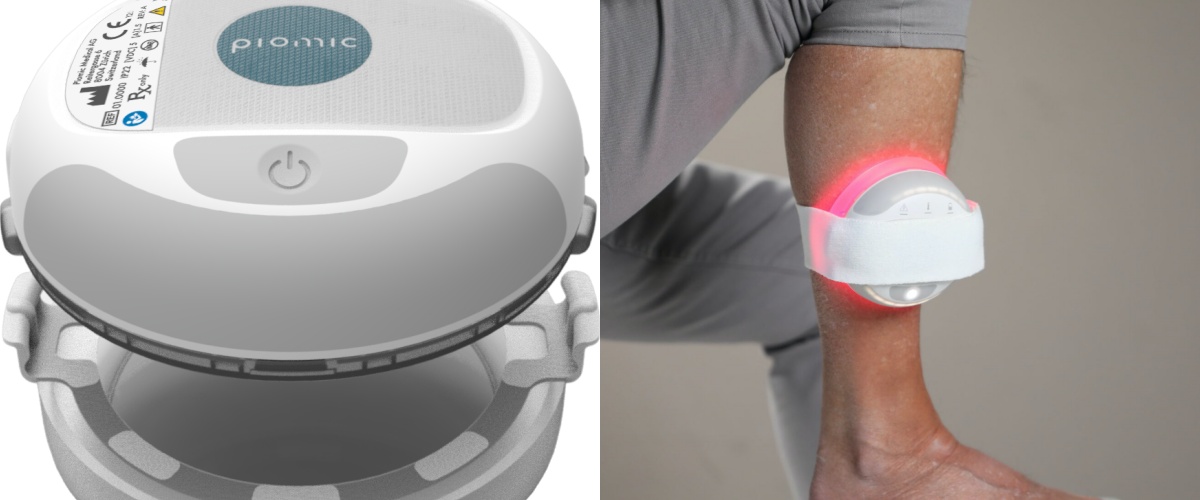
Zurich-based Piomic Medical develops the COMS One Therapy System, a portable, hand-held device embodied with optical and magnetic stimulation technologies to promote wound healing in chronic leg and foot ulcers. The single-button operated device is CE certified, and it has already been used in over 1000 therapies.
With up to 1 % of the population affected, hard-to-heal wounds remain a major challenge for modern healthcare systems, generating some €100 billion in annual direct health care costs worldwide. Piomic, with its new COMs One therapy, addresses a USD 4.8 billion advanced wound care medical devices market. This market is filled with a wide range of solutions such as cold plasma, negative pressure and ultrasound. However, these solutions are limited by performance and portability, and they do not facilitate patient self-management.
Founded in 2016 by Christopher Hertz (CEO), Jari Kruth (CTO), Jürg Fröhlich and Marc Walter, Piomic Medical developed the COMS therapy intended to promote wound healing in the chronic leg and foot ulcers. The system – consisting of a wearable compact, single-button, water-resistant and battery-powered device – incorporates optical and magnetic stimulation technologies, supporting physiological wound healing processes. It integrates the same technology that was previously limited to large and expensive.
The device also fits seamlessly into healthcare professionals’ workflow to allow for a timely transfer of patients from stationary facilities to less expensive out-patient or home setting environments. This is especially relevant since more developed countries seek means to reduce healthcare costs through a timely transfer of patients from stationary settings to less expensive post-acute care settings, such as the out-patient or home care setting. Furthermore, the device follows market mega-trends for portability and patient self-management and integrates into the entire patient journey with applications in stationary, ambulant and home care facilities.
After reaching the CE certification last year in May, the COMS One therapy system is currently being marketed in selected European Union countries. With over 1000 successful COMS therapies already performed on hard-to-heal wound patients, Piomic is also onboarding and finalising the registration of additional selected distributors from other global advanced wound care markets. The next phase will also see the launch of a large randomised controlled trial (RCT) to strengthen further the body of evidence for clinical efficacy and file for the US market clearance via the De-Novo pathway – preparations are underway.
Growing traction
Piomic and its nine employees have remained on a steady growth course supported by a diverse group of investors, including angel investors, employees, clinicians, and board members who participated in multiple subsequent funding rounds. The company is currently raising funds to accelerate market entry and conduct a large randomised controlled trial in the United States.
Support from Innosuisse
Having obtained the Innosuisse Certificate, Piomic is now designated as ready for sustainable growth. During the Innosuisse start-up coaching programme, Piomic worked intensively with the Innosuisse coaches Heiko Visarius, Andreas Emmendörffer and Jean-Pierre Vuilleumier to improve its business concept to make it economically viable. “Lectures and coaching with experts further enabled us to receive valuable support on a range of topics from strategy development, setting up the organisation to market entry. Legal questions regarding intellectual property protection, contracting and taxes were also answered. This exchange helped us significantly to prepare the company for sustainable growth and market entry”, said Christopher Herz, Co-founder and CEO of Piomic.























































Please login or sign up to comment.
Commenting guidelines