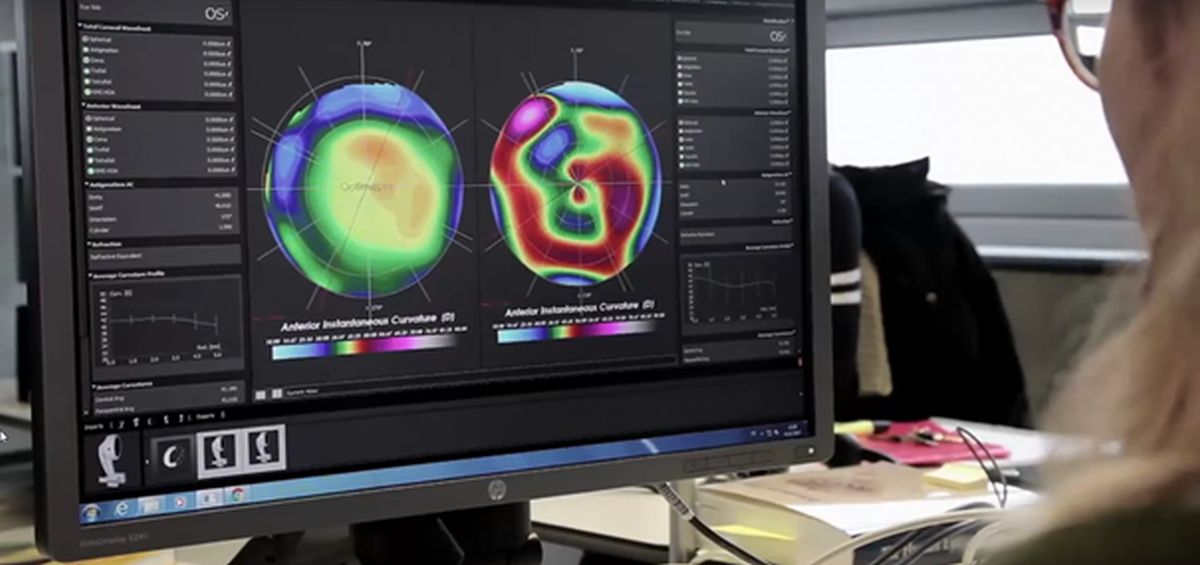
Optimo Medical developed a software solution «Optimeyes» to facilitate personalised eye surgeries. The solution has received the CE mark as a class IIb medical device. The certification gives the startup a green light for daily clinical use in Europe.
The number of eye surgeries conducted worldwide has continued to grow. In Europe alone, 680 Lasik (laser-assisted in situ keratomileusis) surgeries have been performed this year and the number is expected to grow to 757 by 2020. In the England, more than 330’000 catarat surgeries are performed annually and worldwide patients have different other options for corrective eye surgeries.
However, there is still a major challenge: low treatment planning prior to the surgeries and no high-tech solution to facilitate it, which results in low quality results. Optimo Medical seeks enhance results in eye surgery. The startup developed a software solution, “Optimeyes” to establish new standards in eye surgical planning to allow for precision eye surgery and personalised medicine.
Optimeyes makes a full 3D copy of the patient’s eye inside the computer to run different configurations of the surgery. The software predicts the influence of physical interventions in the cornea in a patient-specific way, thus allowing it to simulate and evaluate treatment results in the intervention-planning phase. Furthermore, it enables surgeons to know exactly where to place the incisions ensuring that surgical procedures of the human cornea are safer and more precise.
During the third quarter of 2017, Optimo Medical launched the first version of its solution as a class IIb medical device. Some clinics in Switzerland and Austria have already installed Optimeyes and thanks to the support of its network in the DACH region, the startup plans to bring its solution more clinics in the DACH region. In addition, the company continues to rely on its network of key opinion leaders (KOLs) to promote and develop its innovative technology.
The receipt of a CE marking will furthermore enable the startup to implement its plans, now that it has been approved as ready for daily clinical use within the European market. The CE marking is a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area (EEA).
“With the CE certificate and the market approval for Optimeyes, Optimo Medical AG has reached the most important milestone in the company's history. My team and I are proud of what we have achieved and are now looking forward to the next phase of growth and market attraction”, says Harald Studer, CEO of Optimo Medical.
(RAN)























































Please login or sign up to comment.
Commenting guidelines